32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
5 (684) · $ 22.50 · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

Hydrogen Oxygen Fuel Cells - an overview

3 g of H2 react with 29 g of O2
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
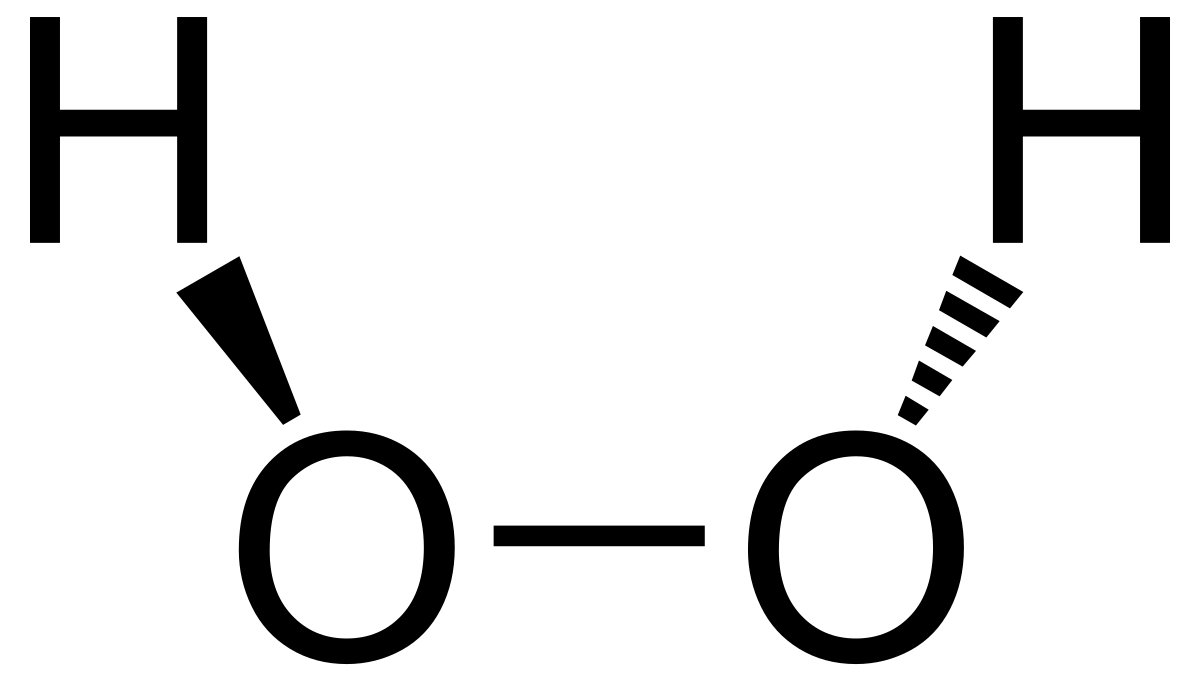
Hydrogen peroxide - Wikipedia

iii. Mass of mathrm{CO}_{2} remaining =319 mathrm{g} Q.88. 6 mathrm{g} of mathrm{H}_{2} reacts with 32 mathrm{g} of mathrm{O}_{2} to yield water. Which is the limiting reactant? Find the mass of water produced

How many grams of water are produced if we react 3 moles of

SOLVED: Question 1: CH4 + 2 H2O → 4 H2 + CO2 Given 80 g of CH4
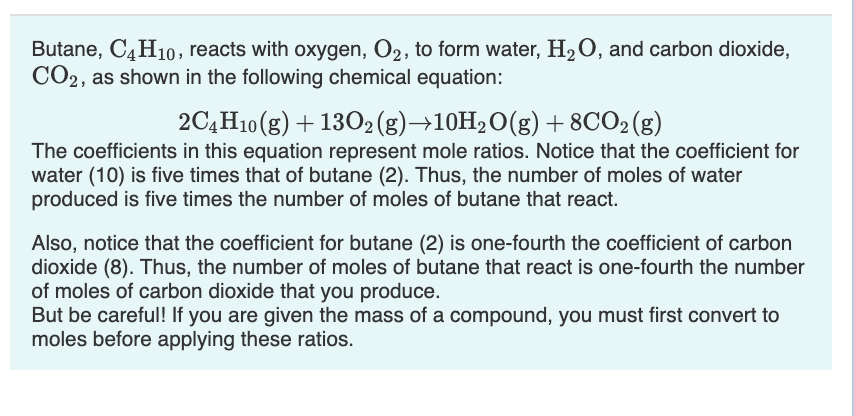
Solved Butane, C4H10, reacts with oxygen, O2, to form water

Glucose Oxidase Type VII, lyophilized powder, main = 100,000units

80 g of `H_(2)` is reacted with 80 g of `O_(2)` to form water
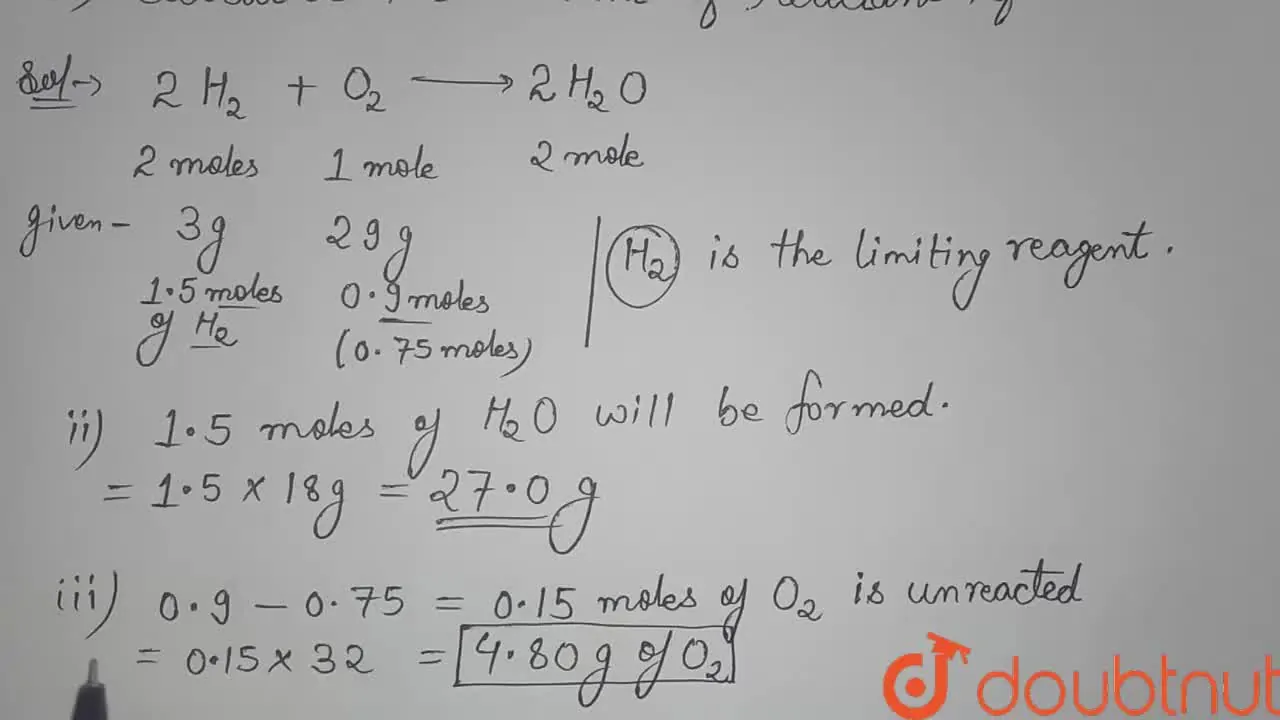
3.0 g of H(2) react with 29.0 g of O(2) yield H(2)O. (i) Which
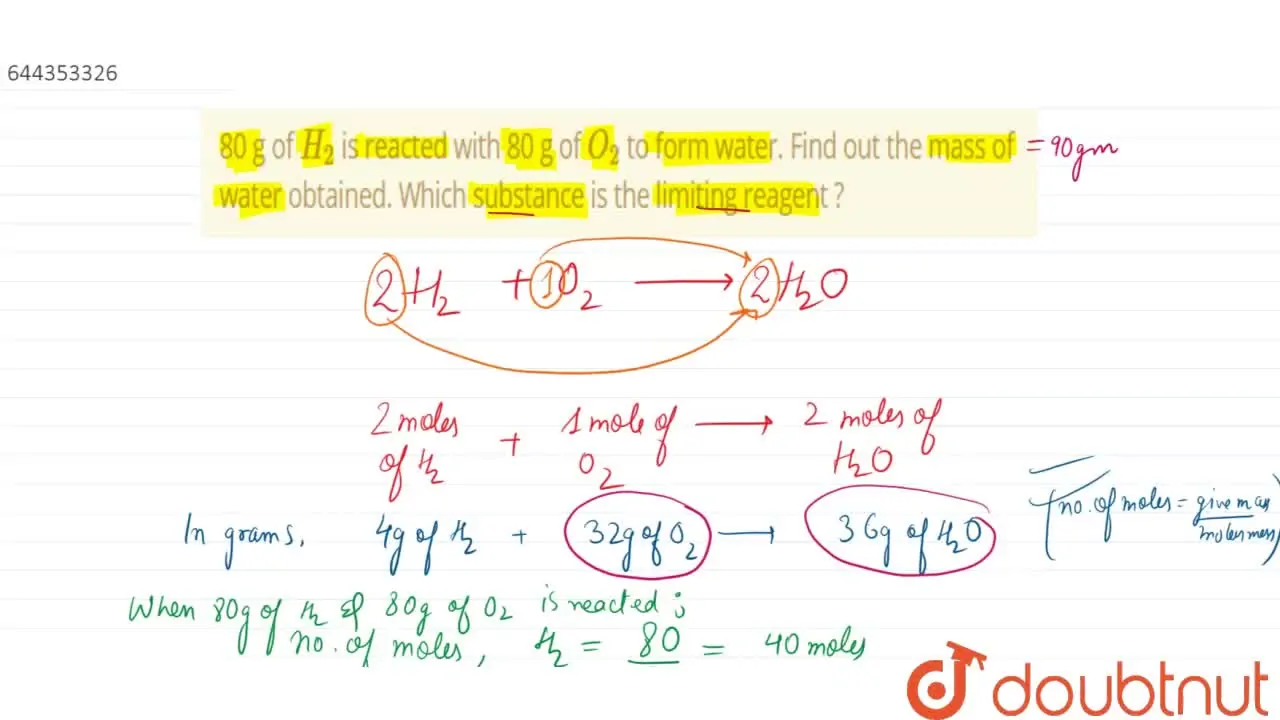
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the

How much mass of water is obtained by reacting 80 g each of

80g of H2 is reacted with 80g of O2 to form water; what are the

3.0 g of H_(2) react with 29.0 g O_(2) to yield H_(2)O (i) What is