FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice
4.6 (248) · $ 9.50 · In stock

The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

MiniMed™ Paradigm™ REAL-Time 522/722 Insulin Pump - User Guides

Diabetes: Medtronic warns on insulin pump overdose
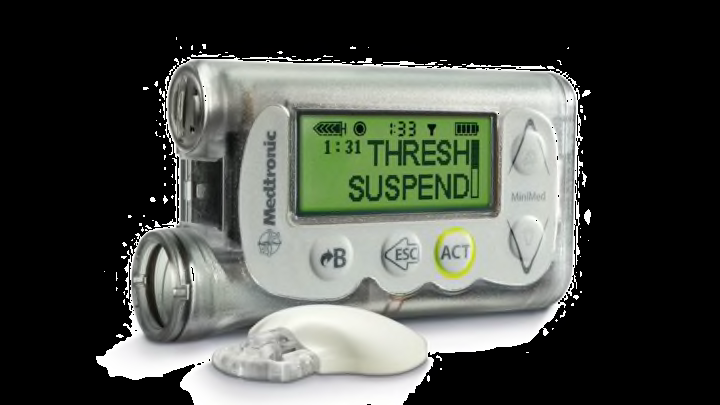
The FDA Is Recalling Medtronic Insulin Pumps Over Hacking Concerns

Medtronic Recalls Some Insulin Pumps as FDA Warns They can be

ICU Medical's Smiths Medical has a Class I infusion pump recall

FDA recalls insulin pumps tied to over 2,000 injuries and 1 death

Stream episode Tandem integrates Dexcom G7 into insulin pumps

Medtronic expands 2 Class I recalls of MiniMed insulin pumps

Erica Scott - CSAM on LinkedIn: Abbott integrates CGM into
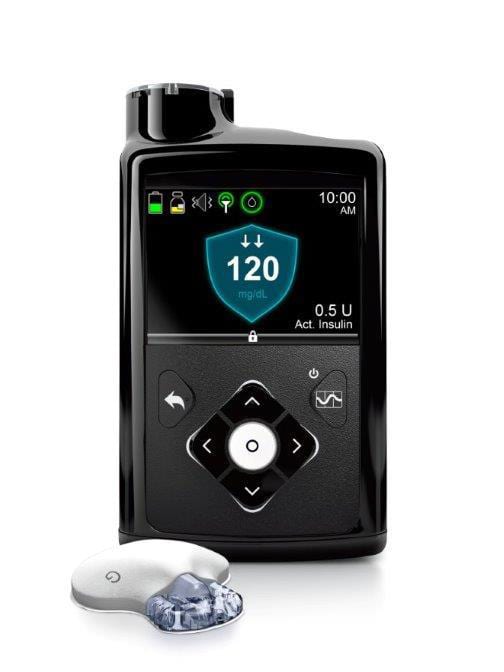
Medtronic recalls some insulin pumps that could lead to dangerous incorrect dosing