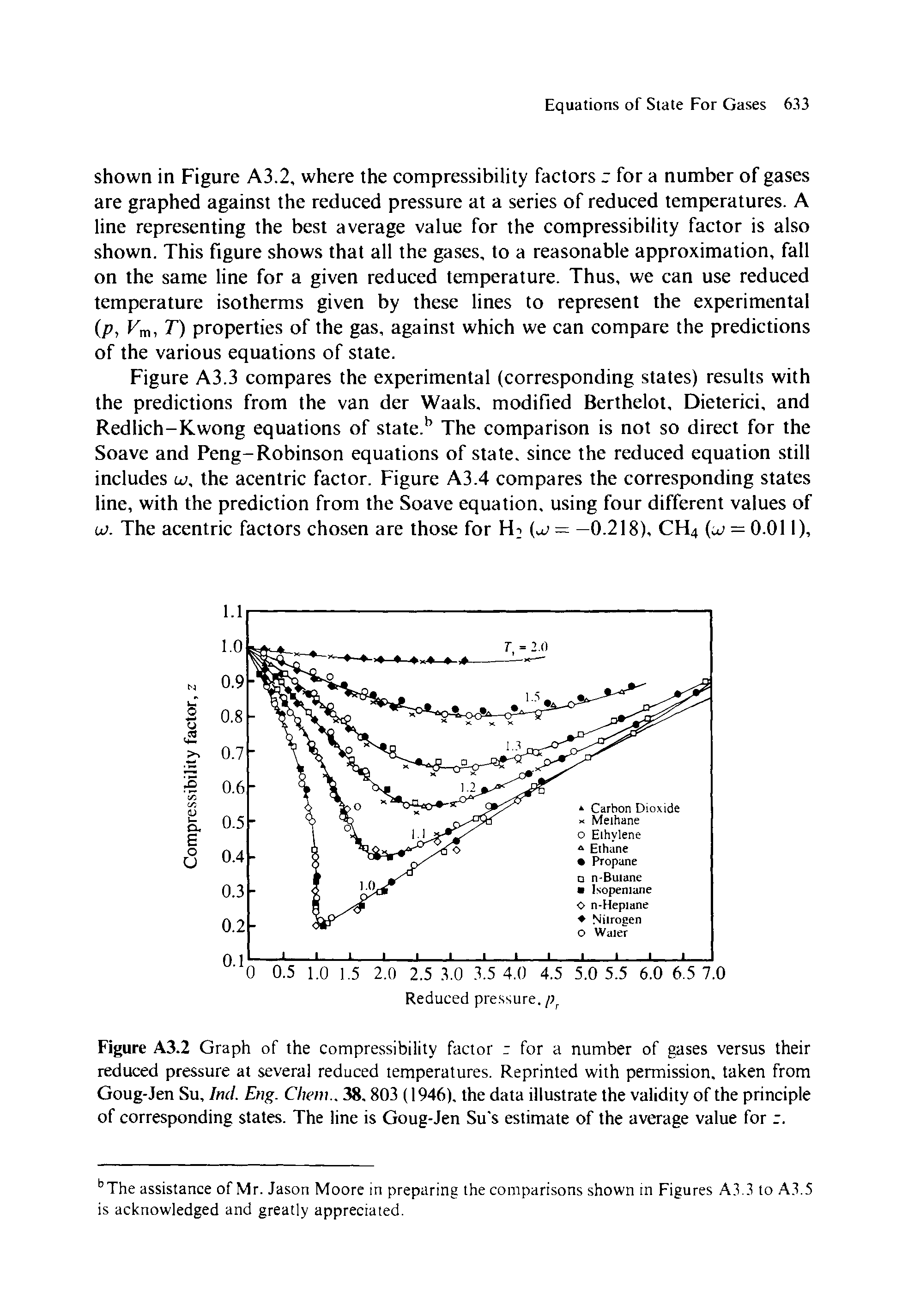
Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
4.8 (374) · $ 14.00 · In stock


Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

Solved The van der Waals equation of state can be used to
Why can't anymore atoms enter in the excluded volume (region of 2 atoms) in the volume correction given by van der Waals equation? - Quora
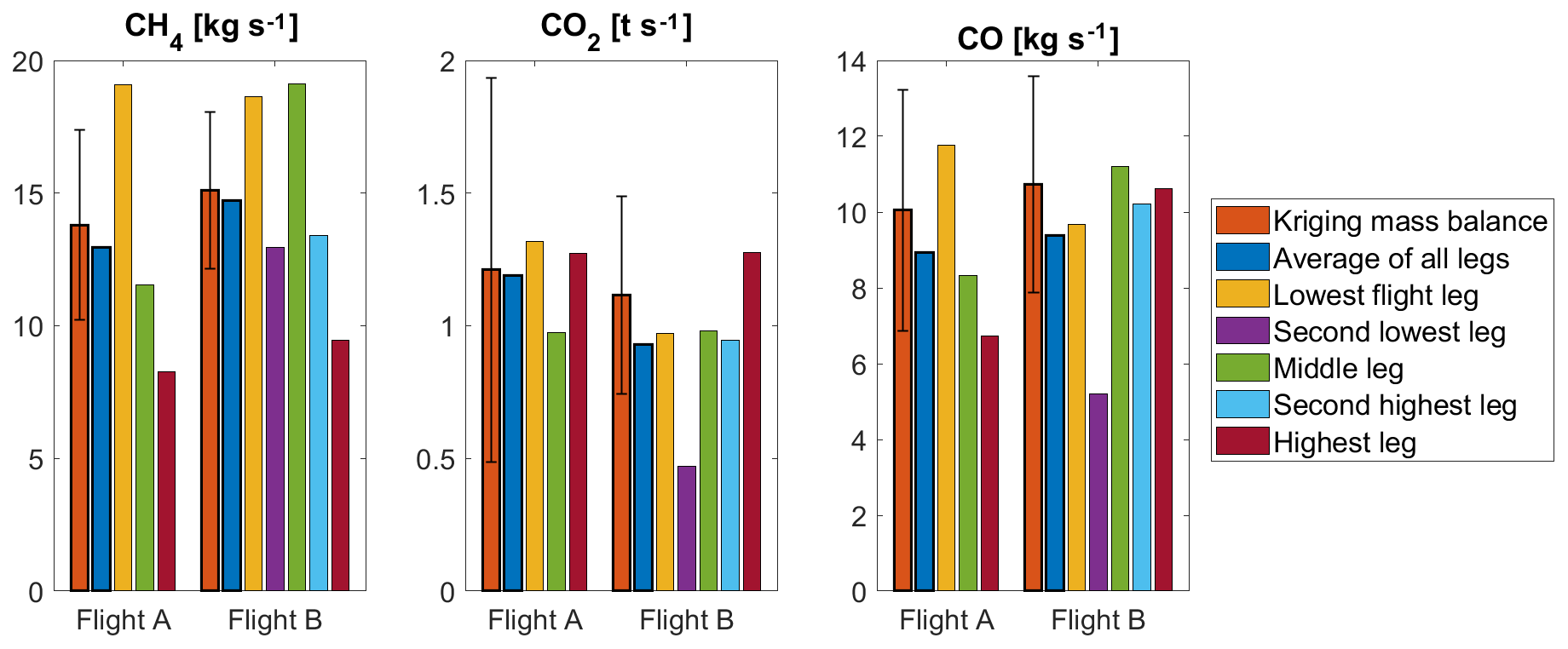
ACP - Estimating CH4, CO2 and CO emissions from coal mining and industrial activities in the Upper Silesian Coal Basin using an aircraft-based mass balance approach

1148 questions with answers in GAS


The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of

Welcome to Chem Zipper.com: A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is
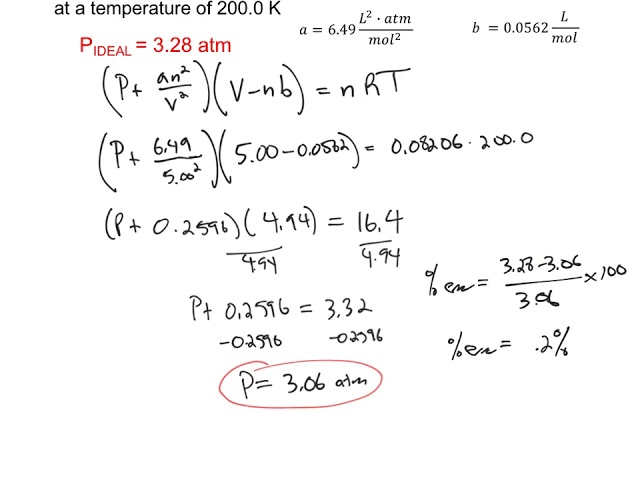
van der Waals example