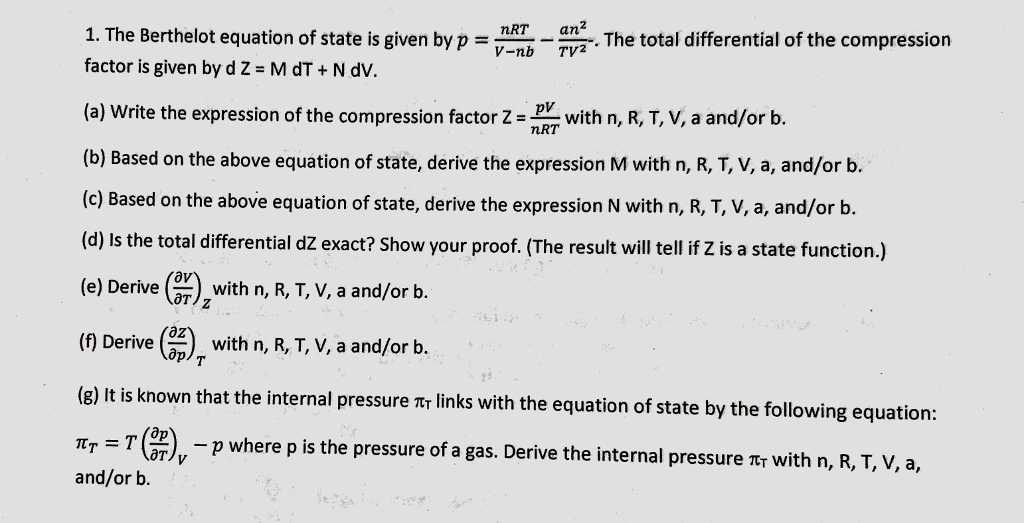
Show that the van der Waals equation leads to values of Z <
4.7 (194) · $ 13.50 · In stock


Van der Waals force - Wikipedia
![CH-Physical Chemistry(8th ed)[英语]Atkins](https://img.yumpu.com/38775605/1/500x640/ch-physical-chemistry8th-edatkins.jpg)
CH-Physical Chemistry(8th ed)[英语]Atkins
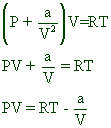
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Van Der Waals Equation of State - an overview

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
![Physical Chemistry: Thermodynamics, Structure, and Change [10 ed.] 9781429290197, 1429290196](https://dokumen.pub/img/200x200/physical-chemistry-in-brief.jpg)
Physical Chemistry: Thermodynamics, Structure, and Change [10 ed.] 9781429290197, 1429290196
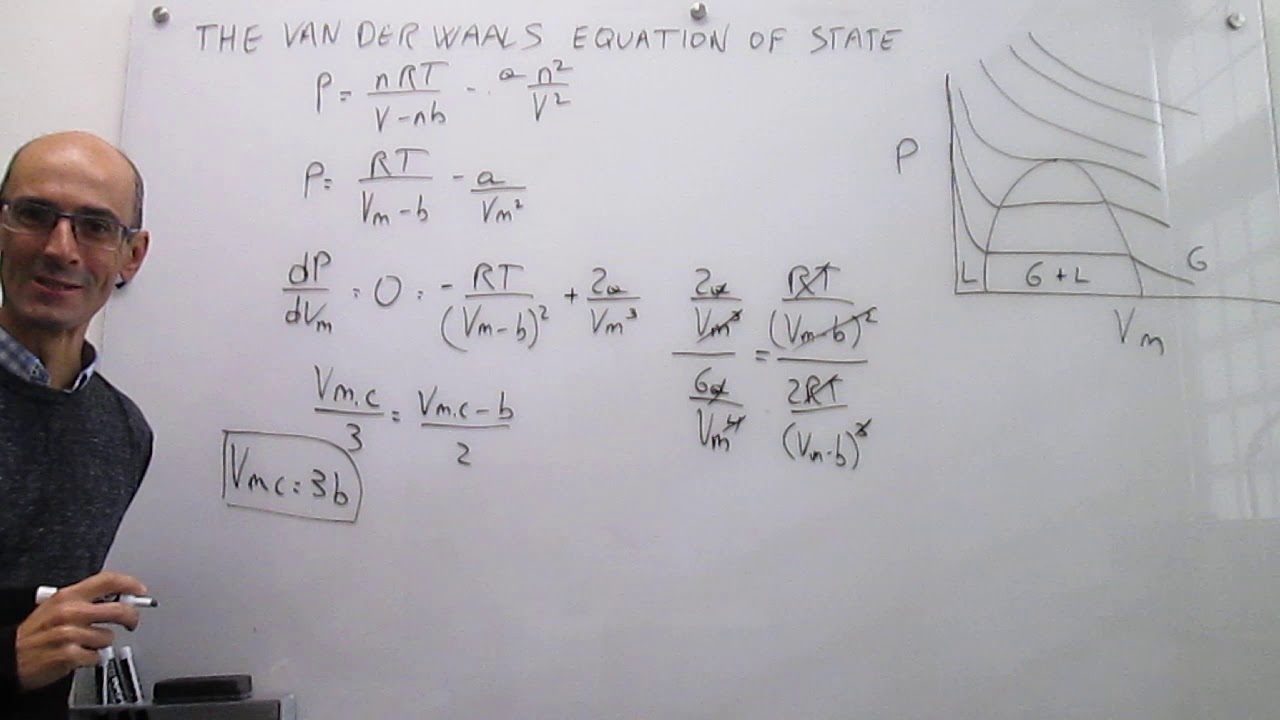
The van der Waals equation of state at the critical point

Gas compressibility factor Z: Ideal gas vs Real gas

Chapter 1 Questions 8th Ed., PDF, Gases
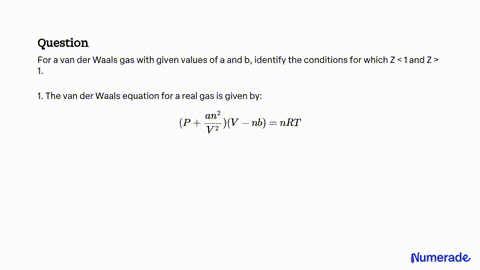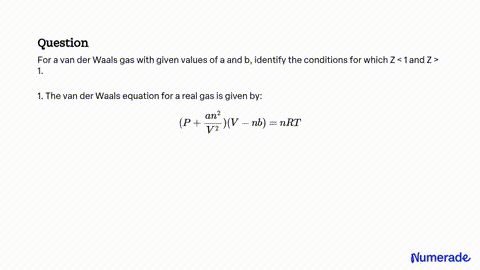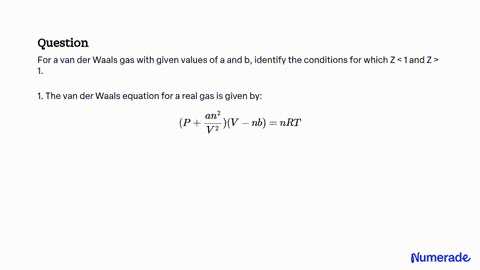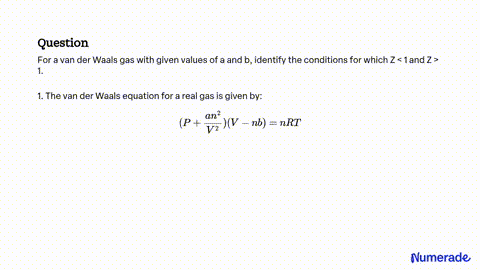
SOLVED: For a van der Waals gas with given values of a and b, identify the conditions for which Z<1 and Z>1

Peter Atkins Julio de Paula Ron Friedman Physical Chemistry Quanta (0358-0408), PDF, X Ray Crystallography

SOLVED: Show that the van der Waals equation leads to values of Z (compressibility factor) < 1 and Z > 1, and identify the conditions, t is, how the temperature T is related to the molar volume Vm, for which these values are obtained? (20 pts)
Why does the van der Waals equation have one positive and one negative correction term? - Quora
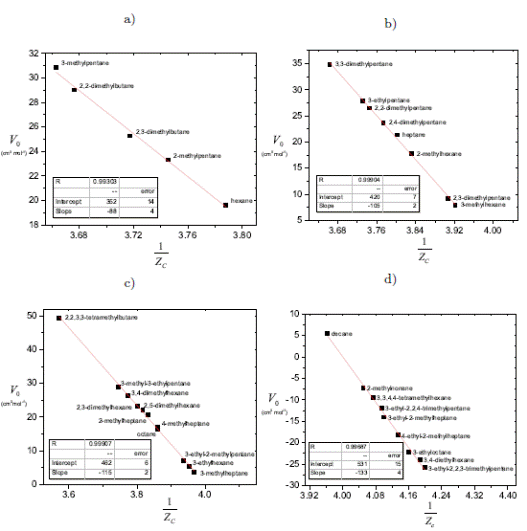
Critical Constants Correlation from van der Waals Equation
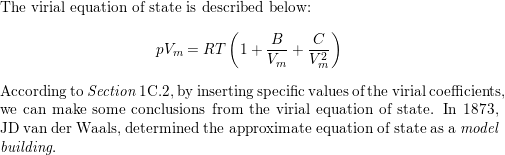
Describe the formulation of the van der Waals equation and s