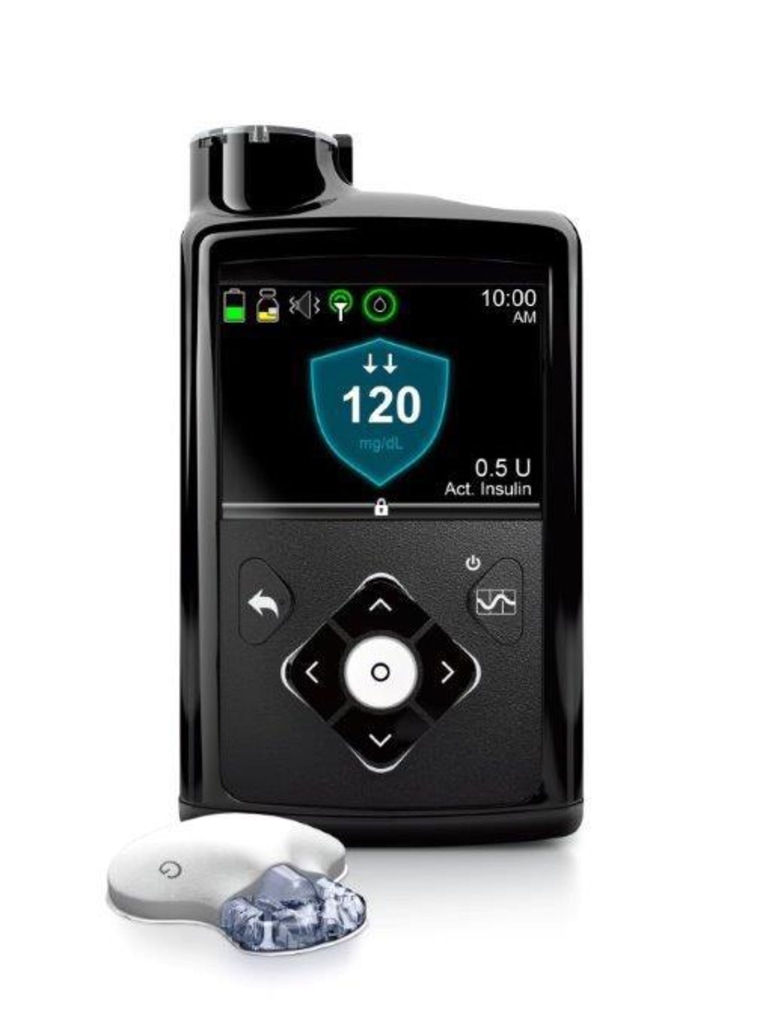
Understanding the Insulin Pump Recall
4.5 (306) · $ 9.99 · In stock
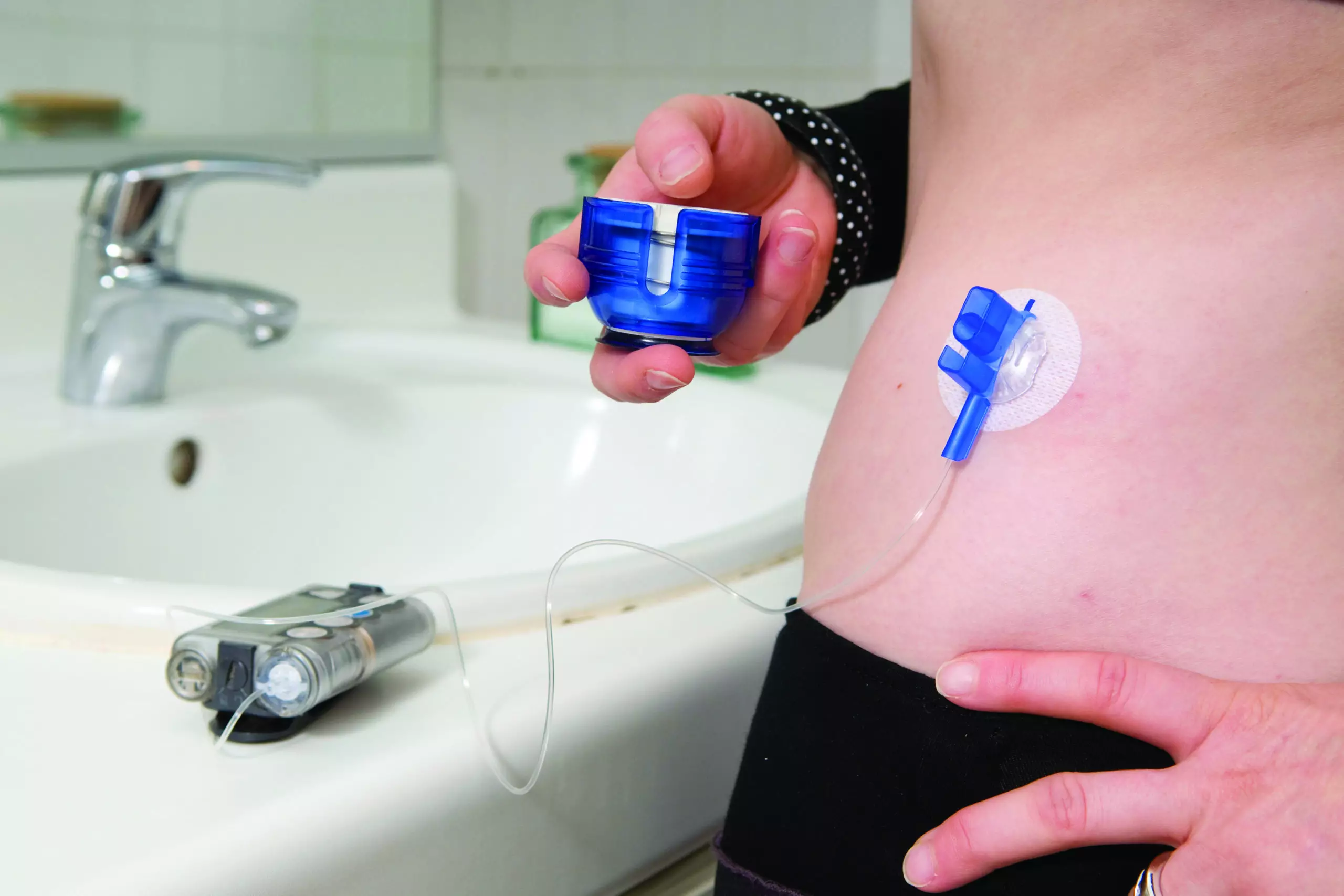
Medtronic is recalling defective MiniMed insulin pumps that could cause severe hypoglycemia or hyperglycemia resulting in seizures, coma and even death

Insulet has a Class I recall for the Omnipod 5 Android App

FDA Product Recall: Medtronic Recalls MiniMed Insulin Pumps for Incorrect Insulin Dosing
Medtronic urgently recalls insulin pump controllers over hacking concerns

Insulin Pump Lawsuit - Mass Torts Central

Understanding the Insulin Pump Recall

FDA Recalls MiniMed Insulin Pumps Due to Injury and Death - Diabetic Nation

Medtronic MiniMed Insulin Pump Injury Recall & Lawsuit

J&J alerts patients to insulin pump cybersecurity flaws, but says risk is low

Medtronic Warns of Stuck MiniMed Pump Buttons

Life-threatening MiniMed 600 Series Insulin Pump dosage issues lead to FDA warning letter over quality control - News