SOLVED: A 35-g ice cube at 0.0 °C is added to 110 g of water in a 62-g aluminum cup. The cup and the water have an initial temperature of 23 °C. (
4.6 (577) · $ 28.50 · In stock
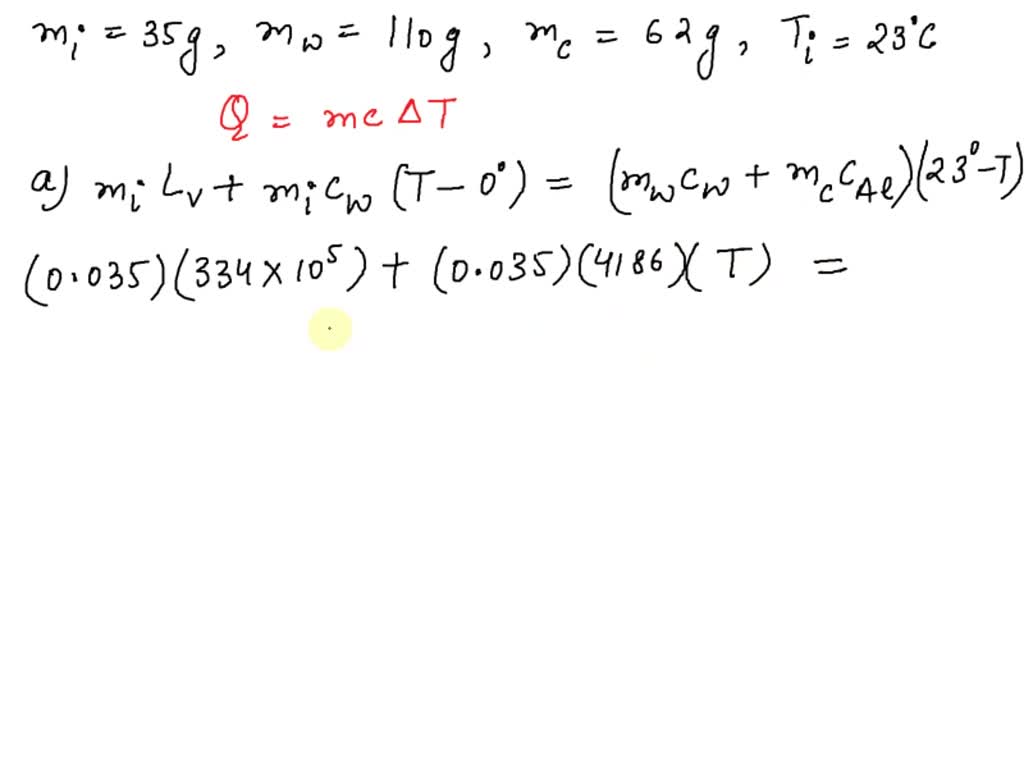
VIDEO ANSWER: Hello students to solve the given question: let us use the equation of heat transfer that is equal to m c c. Here is the specific heat capacity multiplied by delta t that is, temperature difference now, using this relation? Let us solve
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
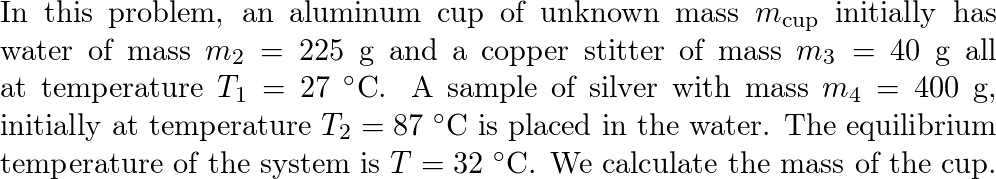
An aluminum cup contains 225 g of water and a 40-g copper st
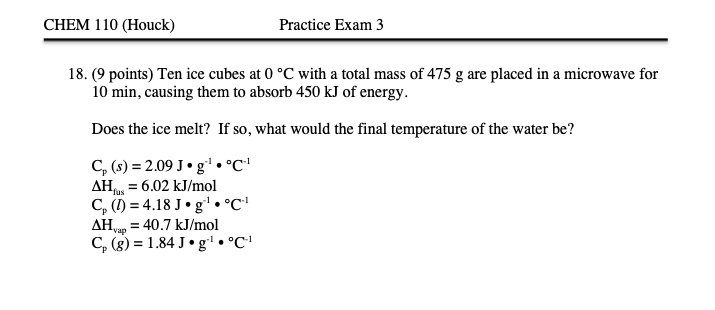
Solved 8. ( 9 points) Ten ice cubes at 0∘C with a total mass
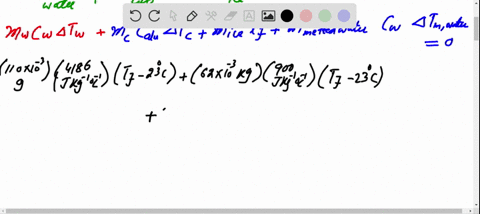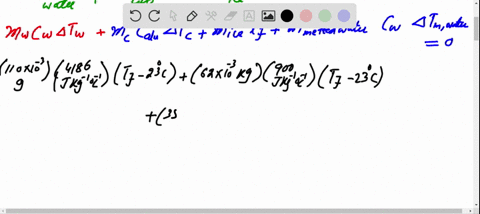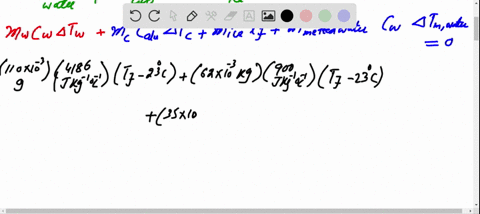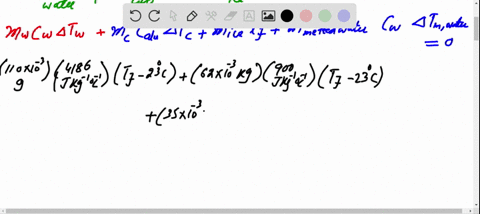
⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added
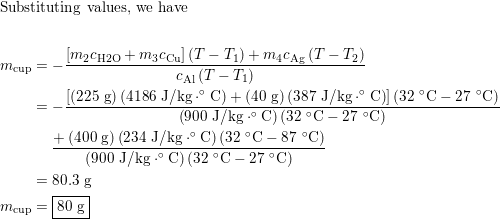
An aluminum cup contains 225 g of water and a 40-g copper st
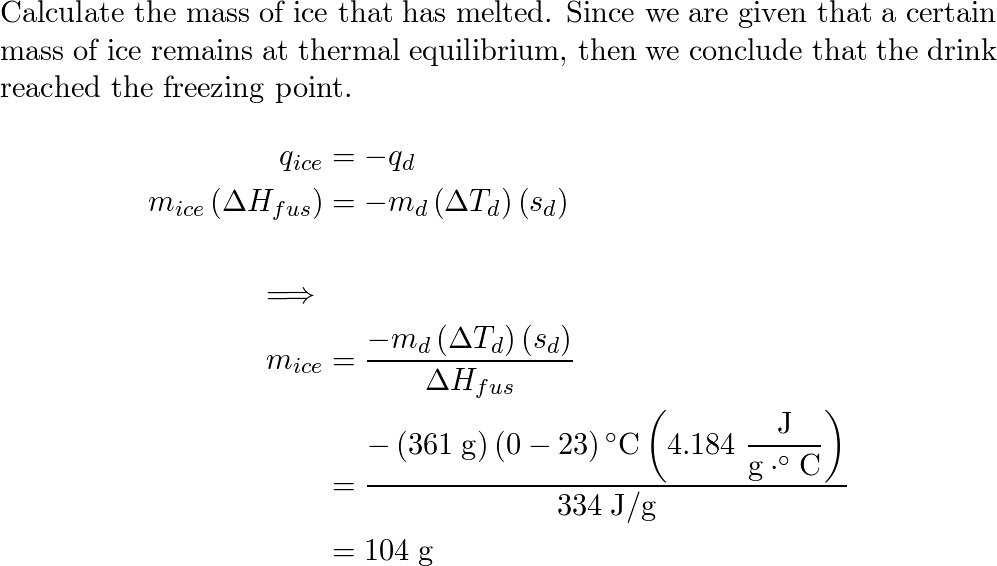
Ice at 0 °C is placed in a Styrofoam cup containing 361 g of

5.22 A 70.0-g piece of metal at 80.0 °C is placed in 100 g of


Final Temperature of Ice and Water Mixture - How Many Grams of Ice

Calorimetry - Chemistry

10 gm of ice cubes at 0°C are released in a tumbler (water equivalent 55 g) at 40°C. Assuming that
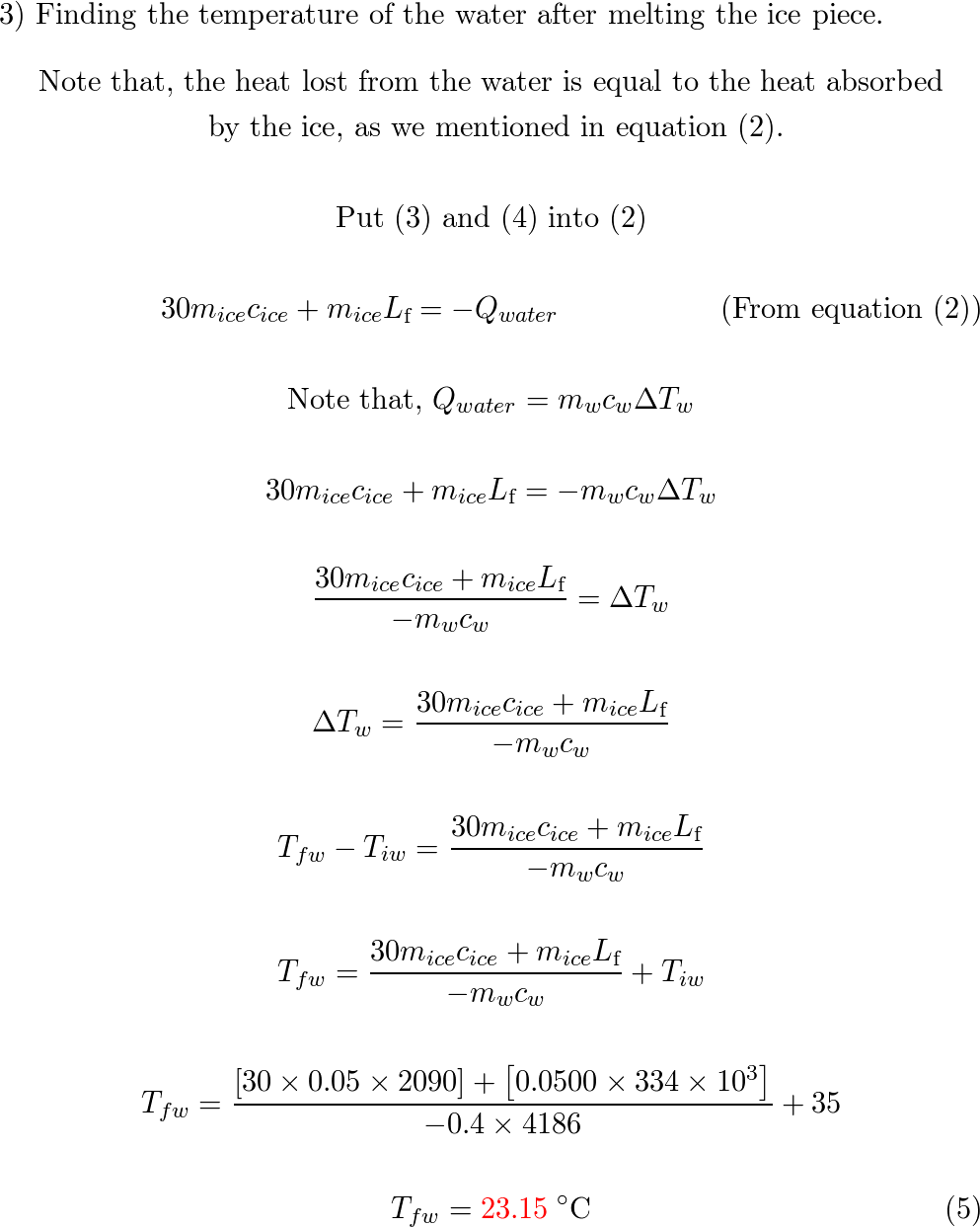
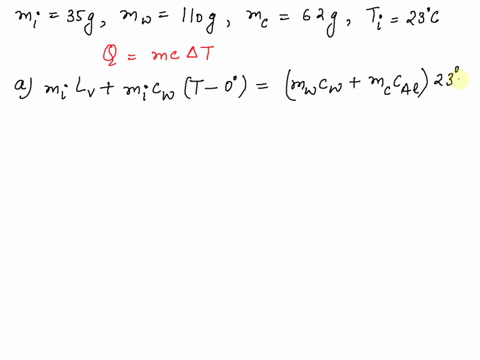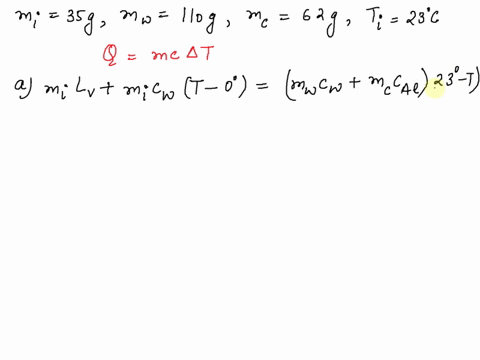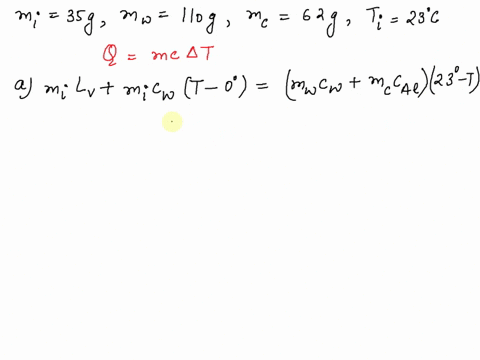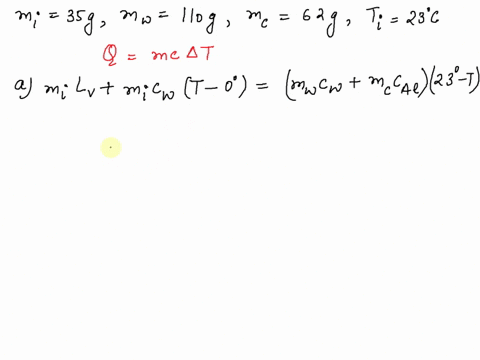
⏩SOLVED:Predict/Calculate A 35- g ice cube at 0.0^∘ C is added
2 kg of ice at 0°c is mixed with 8 kg of water at 20°c. What is







